High patient safety and revascularization efficacy. For the successful prevention and treatment of coronary restenosis.
Restore demonstrates an unparalleled product finish, which retains Paclitaxel in its balloon surface matrix coating. A premature debonding of the Paclitaxel during catheter manipulation and the risk of unintentional cath lab contamination is thereby eliminated.
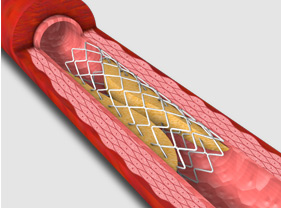
1. Coronary In-Stent restenosis
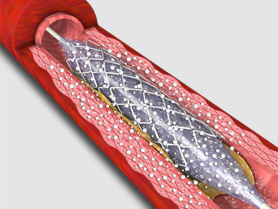
2. RESTORE DCB dilatation of in-stent restenosis
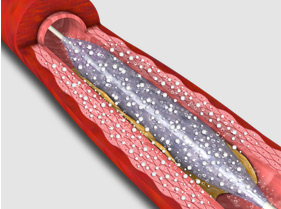
3. RESTORE DCB dilatation of small coronary arteries (ø < 2.3 mm) and bifurcated sidebranch lesions.
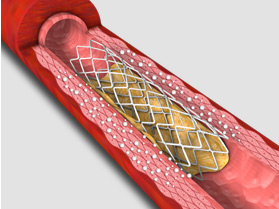
4. RESTORE DCB optimizes coronary stenting results.
‘RESTORE DCB treated arteries show no noticeable coating- induced micro emboli!’
Pre-clinical study report, CV-PATH Institute,
Gaithersbourg USA, Medical Director Renu Virmani MD
Safe Drug Coating Technology
for advanced treatment quality.
SAFEPAX. with the unsurpassed non-crystalline coating and no PTX particulates protect from micro- embolization.
RESTORE ‘SAFEPAX’ technology utilizes the clinically important, stable amorphous PTX drug-coating. RESTORE ‘SAFEPAX’ coating is a step beyond contemporary first and second generation DCB coatings which had to compromise on vulnerable balloon coating mixtures out of a high water soluble drug excipient with relatively large PTX crystals.

Microscopy of the LEGFLOW DCB balloon surface
showing no visible PTX particulates
Minimizing the risk of micro-embolization.
RESTORE DCB ‘SAFEPAX’ technology provides maximum protection from downstream effects to minimize the risk potential of miocardial vascular changes. The biological ‘SAFEPAX’ drug release matrix avoids the side effects of plasticizer or contrast media drug excipients. Coronary artery treatment requires the highest degree of patient safety. The protection from necrosis in arterial tissue to minimize the risk potential of late coroanry aneurysm, and minimizing the risk of micro embolization to avoid myocardial damage, caused by vascular changes.

‘Stable’ vs. ‘unstable’ DEB PTX coating
When invisibility equals safety!
- Homogeneous surface composed of smooth amorphous coating:
‘stable solution’ of PTX /Ammonium Salt excipient - Balloon surface coated with white powder of brittle crystalline PTX + excipients
No need of an extra insertion tool. The RESTORE coating integrity. A step beyond.
The indestructible, structural balloon coating integrity, combined with a protective drug coating finesse, does not require the use of an extra insertion tool.
The RESTORE Paclitaxel balloon surface coating cannot be wiped or fall off the balloon during catheter preparation, insertion or manipulation. RESTORE prevents endangerment of cathlab personnel, and guarantees a safe and predictable drug delivery to the target lesion site.
Proven! Optimal therapeutic drug-in-tissue bioavailability resulting in maximal clinical efficacy.
No signs of vessel toxicity were found. No other safety concerns were noted in animals studied for up to 90 days.*
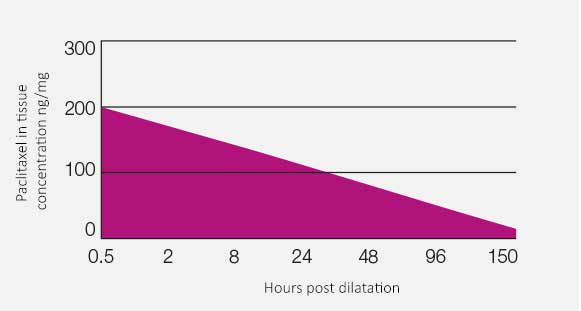
RESTORE DCB drug-in-tissue bioavailability.
A short-term balloon-to-vessel wall contact time of 45 sec. is sufficient to inhibit SMC proliferation sustainably for up to 150 hours.
Pre-clinical results confirm no noticeable embolization results similar to POBA. Pre-clinical study results confirm a most effective drug delivery into the vascular tissue, showing a sustained drug effect of up to 28 days. ‘Cardionovum’s DEB shows great effectiveness in neo-intimal growth reduction in diseased vessels.’
*Source: Report on pre-clinical studies performed
RESTORE SAFEPAX performance. Effective DCB PTX drug-transfer.
Consistent and predictable drug delivery to the artery lesion site results in a homogenous and maximized drug absorption into the arterial tissue.
RESTORE ensures a high patient safety by non-crystalline coating ahead of 2.0-3.0µm large brittle Paclitaxel crystals on most other DCBs.
- Controlled trials confirm the excellent Procedural success rates >98% in both ISR and SVD patients1 3
- No procedure-related complications with RESTORE in two ISR trials and 160 patients: <1% complication rates1 3
- Similar complication rates to DES in vulnerable SVD patients (p=0.19 for comparison)2
- Only DCB with demonstrated low complication rates (~3%) in patients with very small vessels2
References
- Miglionlco M, Manglacapra F, Nusca A, et al. Efficacy and Safety of Paclitaxel-Coated Balloon for the Treatment of In-Stent Restenosis In High-Risk Patients. Am J Cardiol 2016; 116: 1690-4.
- Tang Y, Qlao S, Su X, Chen Y, Jin Z, Chen H, Xu B, Kong X, Pang W, Liu Y, Yu Z, Li X, Li H, Zhao Y, Wang Y, Li W, Tian J, Guan C, Xu B, Gao R, for the RESTORE SVD China ln119stlgators, Drug-Coated Balloon Versus Drug-Eluting Stent for Small Vessel Disease: The RESTORE SVD China Randomized Trial, JACC: Cardiovacular Interventions (2018), doi: https://dol.org/10.1016/j.jcin.2018.09.009.
- Chen Y, Gao L, Qin Q, Chen S, Zhang J, Chen H, Wang L. Jin Z. Zheng Y, Zhang Z, Li H, Li X, Fu G, Chen L, Sun Z, Wang Y, Jin Q, Cao F, Guo J, Zhao Y, Guan C, Li W, Xu B, for the RESTORE ISR China Investigators, Comparison of Two Different Drug-Coated Balbons in In- Stent Restenosis: The RESTORE ISR China Randomized Trial, JACC: Cardiovacular Interventions (2018), doi: https://doi.org/10.1016/J.jcin.2018.09.010.
RESTORE SVD China: RESTORE is a proven equal alternative to zotarolimus-eluting stent in patients with coronary small vessel lesions2
Percent diameter restenosis at 9 months
Non-inferiority proven at p <0.001
No difference in 12-months rates
of target lesion failure between
RESTORE and zotarofimus-eluting
stent (p=0.47)
Multi-centre, randomised, controlled clinical trial in 262 subjects at 12 sites in China. The primary end point was in-segment diameter stenosis after 9 months follow-up. Major adverse events were evaluated at 1, 6, 9 and 12 months and will be collected up to 5 years.
RESTORE ISR China: RESTORE is a proven equal alternative to the bestin- class DCB in the treatment of patients with coronary in-stent restenosis3
Primary end point: statistically similar rates of in-segment late loss at 9 months
Results were valid tor both in-segment
and in-device late loss at one year
RESTORE matches best-in-class DCB on rates of target lesion failure (TLF) over 12 months
TLF rates with RESTORE
and the best-in-class DCB
Multi-centre, randomised, controlled clinical trial in 240 subjects at 12 sites in China. Patients had in-stent restenosis ≥70% diameter on visual assessment, or ≥50% diameter stenosis and with ischaemic symptoms on coronary angiography.
The primary endpoint was in-segment late lumen loss of the target lesion at 9 months after the procedure. Major adverse events were evaluated at 1, 6, 9 and 12 months.












